Abstract
Background
A significant proportion (up to 37%) of patients (pts) with chronic phase (CP) CML experience either resistance or intolerance to first-line tyrosine kinase inhibitor (TKI) therapy within 5 years and transition to other treatment options(Heeg B, et al. 2021).
There are limited data regarding experiences and unmet needs of pts receiving second and later TKI therapy for CML, particularly with respect to quality of life and emotional wellbeingand concerns around disease response and long-term outcome. The CML survey on unmet needs (SUN) research aims to further characterize in depth these unmet needs from the perspective of both the pts and treating physicians.
Methods
CML SUN consists of two sequential phases. Phase 1 entailed of 60-minute in-depth telephone qualitative interviews with adult pts on second line or later TKI treatment and physicians treating CML in France, Germany, Japan and the USA. The insights from this initial phase will inform the design of the second phase, a larger quantitative survey. This abstract reports the results of the Phase 1 qualitative research.
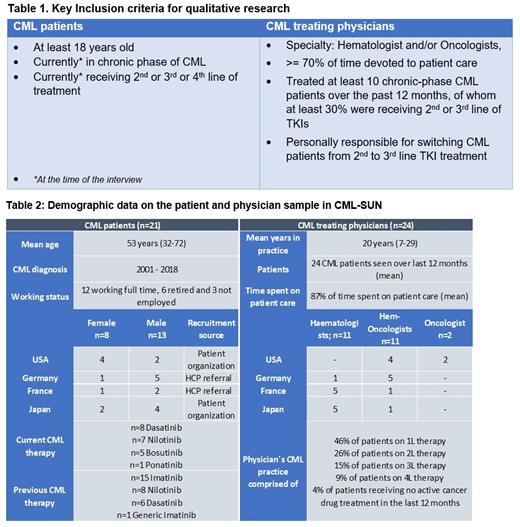
Pts were recruited through physician referral, pt panels, pt advocacy groups and social networking service sources, while physicians treating CML were recruited via panels/databases. Key inclusion criteria can be found in Table 1.
A semi-structured interview guide covering the same topics for both pts and physicians was used to conduct the interviews. The guide consisted of pre-determined but open-ended questions on the topics of perception of the disease, burden of disease, treatment journey, the impact of treatment change, adverse events (AEs), ideal treatment and needs which have not been previously identified or met.
For each of the recorded interviews, a detailed content analysis was conducted to organize the collected data by themes and topics. This content analysis was used to compare responses between individuals within and across countries, and used to identify insights and trends.
Results
A total of 21 pts and 24 physicians were interviewed (Table 2: demographics).
Physician: While physicians were mostly the ones to initiate treatment switch discussions with their pts, they typically did not provide pts with different treatment options and/or clear treatment goals when selecting 2 nd line or subsequent therapy. Most believed that pts & caregivers have minimal or no role in this matter. Therapy switch tended to happen faster if due to lack of treatment response rather than side effects which, unless severe or very frequent, were first treated symptomatically or with dose modification. Physicians felt that pts have minimal or no unmet needs requiring support when it comes to switching therapies.
Patients: An almost equal proportion switched treatments due to resistance vs due to AEs. The need to switch TKI therapy induces anxiety and concerns for pts. Some pts downplayed their AEs either because of fear of triggering a therapy switch or trying to avoid being a burden. Most pts expressed the need to receive additional information on the necessity of therapy switch and on the potential therapy side effects.
While physicians and pts had similar goals for the new treatment: to be effective and generally tolerable; physicians placed a higher priority to the efficacy of the treatment, whilst pts placed a higher priority to reducing the burden of AEs.
Conclusions
This first phase of this research has identified different perspectives and needs between CML pts and physicians in relation to change of TKI therapies, as well as potential divergencies between the two groups regarding their perception of unmet needs.
This information will help design the second phase of the study which will further quantify the impact of these unmet needs in a larger population across a broad geography of 11 countries. This knowledge can be used to raise awareness and to develop support strategies to improve current CML management practices and the pt experience.
Pemberton-Whiteley: Acute Leukemia Advocates Network: Consultancy, Membership on an entity's Board of Directors or advisory committees; Leukaemia Care: Current Employment; AbbVie, Agios, Amgen, Astellas, Autolus, Bristol-Myers Squibb, Celgene, Daiichi Sankyo, Gilead, Incyte, Jazz, Janssen, Kite, Kyowa Kirin, Mallinckrodt, Novartis, Pfizer, Roche, Servier, Takeda: Consultancy, Other: Organizational grant funding, Speakers Bureau; WECAN (Workgroup of European Cancer Patient Advocacy Networks): Consultancy, Membership on an entity's Board of Directors or advisory committees; CML Advocates Network: Membership on an entity's Board of Directors or advisory committees. Cortes: Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Bristol Myers Squibb, Daiichi Sankyo, Jazz Pharmaceuticals, Astellas, Novartis, Pfizer, Takeda, BioPath Holdings, Incyte: Consultancy, Research Funding; Bio-Path Holdings, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Research Funding; Sun Pharma: Consultancy, Research Funding. Clements: Novartis Oncology: Honoraria, Other: Member of advisory board; Takeda Pharmaceuticals: Honoraria; Pfizer Pharmaceuticals: Honoraria, Other: Educational Grant; Leukemia and Lymphoma Society NPO: Membership on an entity's Board of Directors or advisory committees, Other: Patient Financial Assistance; CML Advocates Network NPO / CML Horizons Conferences: Honoraria, Membership on an entity's Board of Directors or advisory committees; The MAX Foundation NPO: Honoraria. Ruiz: Novartis: Honoraria; Pfizer: Honoraria. Rea: Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees. Machado: Pfizer Canada: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Lang: AstraZeneca: Consultancy, Honoraria; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Takahashi: Novartis Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Otsuka Pharmaceutical: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Chugai: Research Funding; Eizai: Research Funding; Asahikasei: Research Funding; Kyowahakko-Kirin: Research Funding; Toyamakagaku: Research Funding; Ono: Research Funding. Deekes: Novartis: Honoraria. Moon: Novartis: Honoraria, Other: Member of advisory board. Grigg: Novartis: Honoraria, Other: Member of advisory board. Steagall: Novartis: Honoraria. Borowczak: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Pilipovic: Novartis: Current Employment, Current equity holder in publicly-traded company. Frank: Novartis: Current Employment, Current equity holder in publicly-traded company. Constantinescu: Novartis: Other: Cristina Constantinescu is an employee of Ipsos, who were paid consultants to Novartis. Boquimpani: Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jansen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pinth Pharma: Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal